Your experimental write-up should include the following:
- Graphs of time/temperature data from all combustion runs.
- Calculation of heat capacity of the bomb using the calibration data; give individual values for each run and the average with standard deviation.
- Calculation of heat of combustion for each compound (individual values and averages) with appropriate corrections for fuse wire.
- Calculation of internal energy and enthalpy of combustion for each compound.
- Discussion of errors relative to literature data. The literature value for the enthalpy of combustion of naphthalene is D H° = 5156.3 kJ/mol.
- Discussion of heats of combustion for varying aromaticity in the naphthalene series.
Corrections
Iron: Remember that you must correct for both the inclusion of the combustion of the iron wire as well as its incomplete combustion.
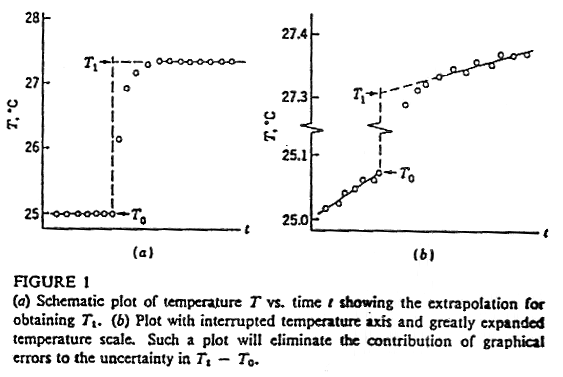
Temperature: The time and temperature readings that you took during each run should be put into table form and graphed. In order to compensate for temperature drift, you will need to fit lines through the points at the beginning and the end of each combustion run, when the temperature has more or less stabilized. (See the diagram below.) The corrected D T value will be used in your calculations. It is the difference between the values of these two lines at the time point when the temperature begins its rapid increase:
Calculations
- Calculate the heat capacity of the calorimeter, Ccal (J/K),
using the following equation:
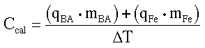 (Eq. 3)
(Eq. 3)where
qBA = heat of combustion of benzoic acid (26435.8 J/g)
mBA = mass of benzoic acid that combusted (g)
qFe = heat of combustion of iron (5858 J/g)
mBA = mass of iron fuse wire that combusted (g)
D T = corrected temperature rise in the calorimeter (K)
- Calculate the heat of combustion of each sample run.
qcombustion = Ccal x D T (Eq. 4)plugging Eq. 3 into Eq. 4 gives: qcombustion = (qs x ms) + (qFe x mFe) therefore:
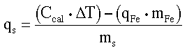
(Eq. 5)
where
qs = heat of combustion of the sample (J/g)
ms = mass of the sample (g)
- Calculate the molar internal energy of combustion of each sample run. Since these processes are constant volume processes the heat of combustion for a sample is the internal energy change for the process. It is necessary to calculate the molar internal energy change for the combustion reaction.
- Calculte the enthalpy change for the combustion of each sample (not including the bezoic acid used for calibration). To determine the molar enthalpy change for each reaction one must write the balanced chemical reaction for each of the oxidation reactions. From the reaction one can determine how the moles of gaseous reactants and products differ for the combustion of one mole of sample. This allows one to determine the D n in eqn 2. D n is negative if there are more moles of gaseous reactants than products, and positive if there are more moles of gaseous products than reactants. Then one can use the D (pV) correction to the molar D U to determine the molar D H for the reaction